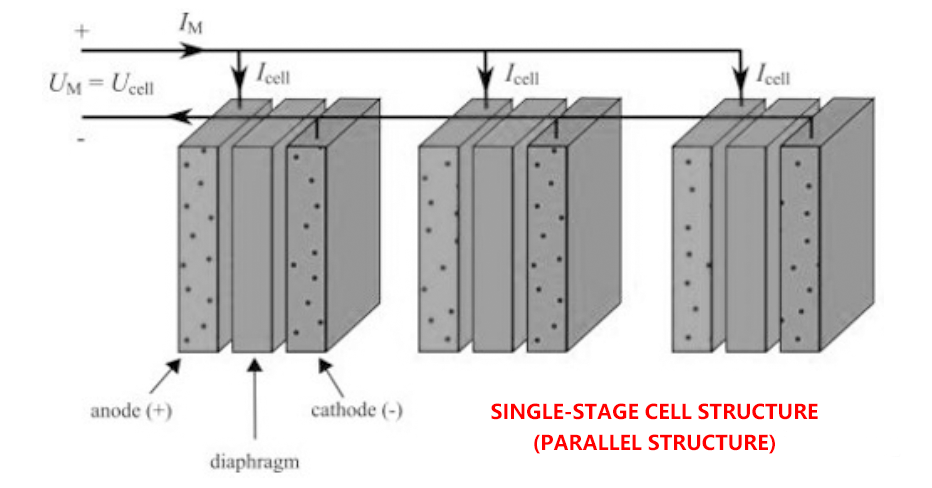
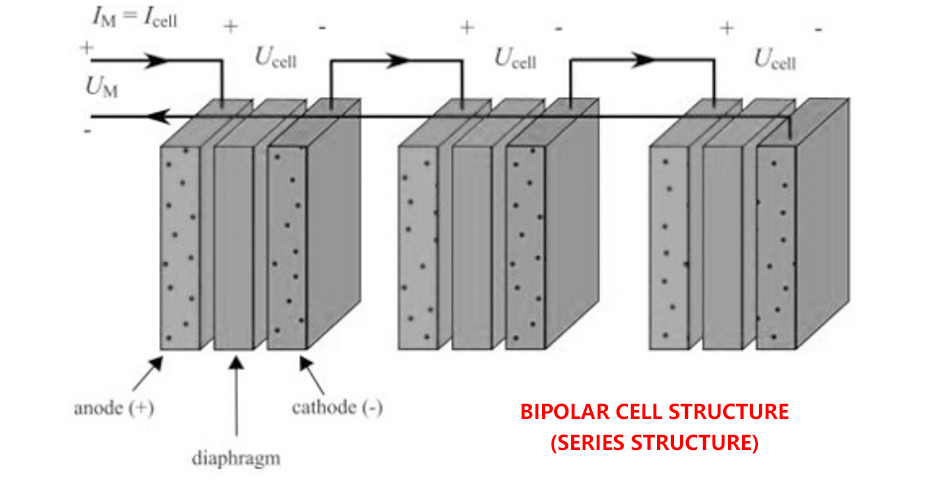
There are usually two basic cell configurations, namely unipolar cell and bipolar cell. Figures 1 and 2 illustrate unipolar and bipolar battery configurations, respectively. UM and IM are the voltage and current of the electrolytic module.
4.Separating Diaphragm or separating diaphragm A separator is used to separate the anode and cathode from each other. Originally, the separator (separation membrane) was made of asbestos, but now asbestos is no longer used due to safety and health regulations. Today, they are dominated by polybenzoimide, polybenzene sulfide, sulfonated polymers and composites. The composite materials are mainly ceramic materials or microporous materials, such as polyether sulfone (PES) a reinforced, microporous polymer film, glass reinforced polyphenylene sulfide (PPS) compounds, titanium oxide mesh on the nickel oxide layer, potassium titanate pores and mainly ceramic. Diaphragms must keep product gases separate to maintain efficiency and safety. In addition, the diaphragm must be permeable to water molecules and hydroxide ions.
8.End Plate: End plate. It is used to fix both ends.
I. Design of single-pole electrolytic cell
In the unipolar structure, each electrolytic cell is connected in parallel to form a large module of electrolytic reactor, as shown in Figure 1. Thus, the voltage between individual electrode pairs is directly equal to the total electrolytic pile voltage, and the sum of the electrolytic pile current is equal to the total battery current. Moreover, in this structure, the same electrochemical reaction occurs on both sides of each electrode. The reaction can be either hydrogen evolution or oxygen evolution, depending on the polarity of the electrodes involved.
1.1. Solidwork design of unipolar alkaline electrolytic reactor
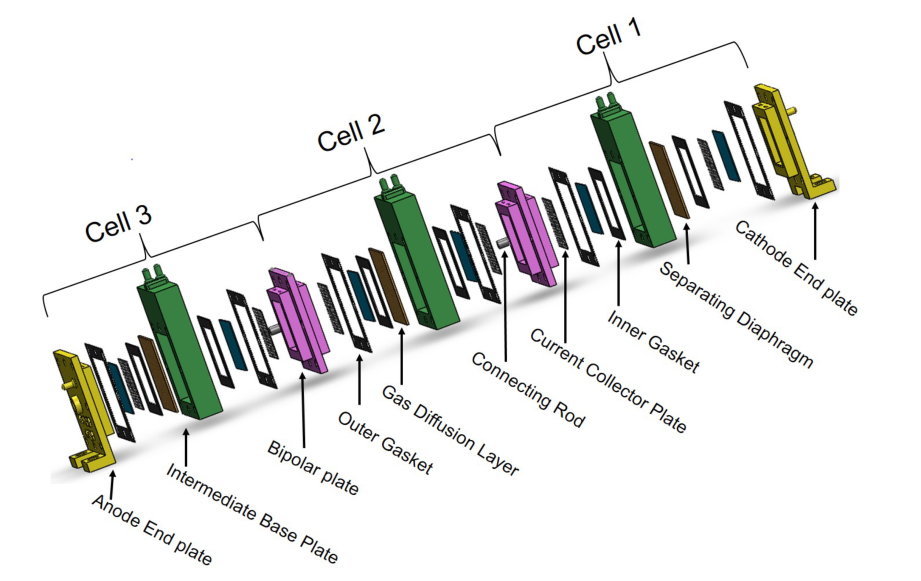
The stack consists of three cells. In addition, in order to improve the electrolytic efficiency, the porous stainless steel collector plate is used instead of the ordinary collector plate, as shown in the figure.
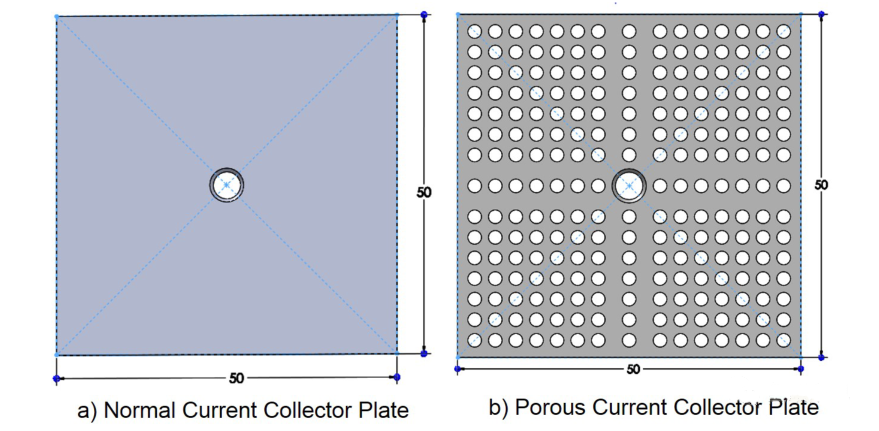
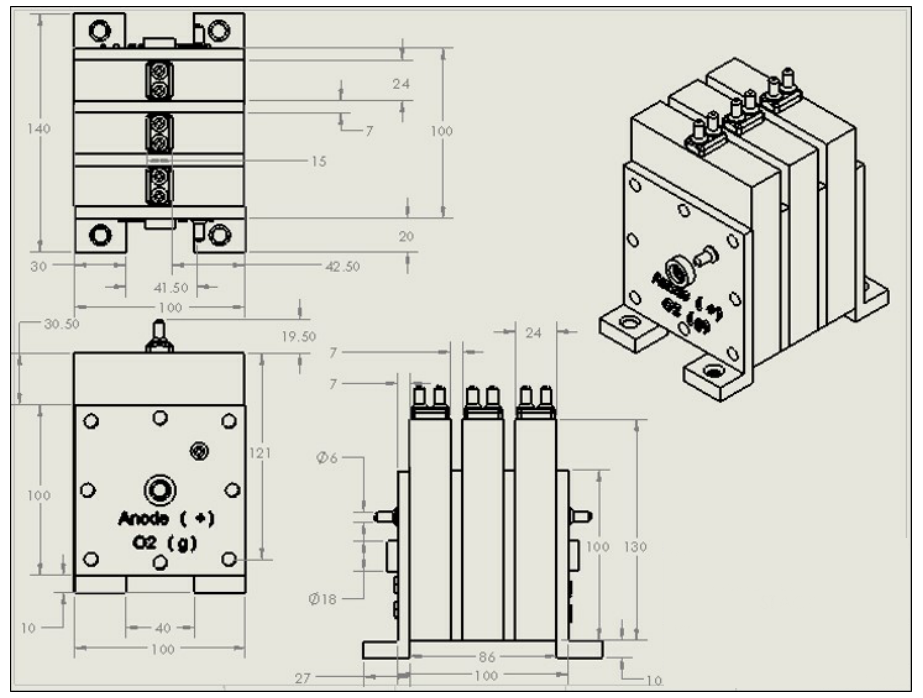
The fabrication of monopolar alkaline electrolytic reactor is an important link to analyze and observe the reactor performance. The bipolar plate and connecting rod are used to connect the battery. The basic components of SolidWorks for unipolar alkaline electrolytic reactor are shown in the figure below.
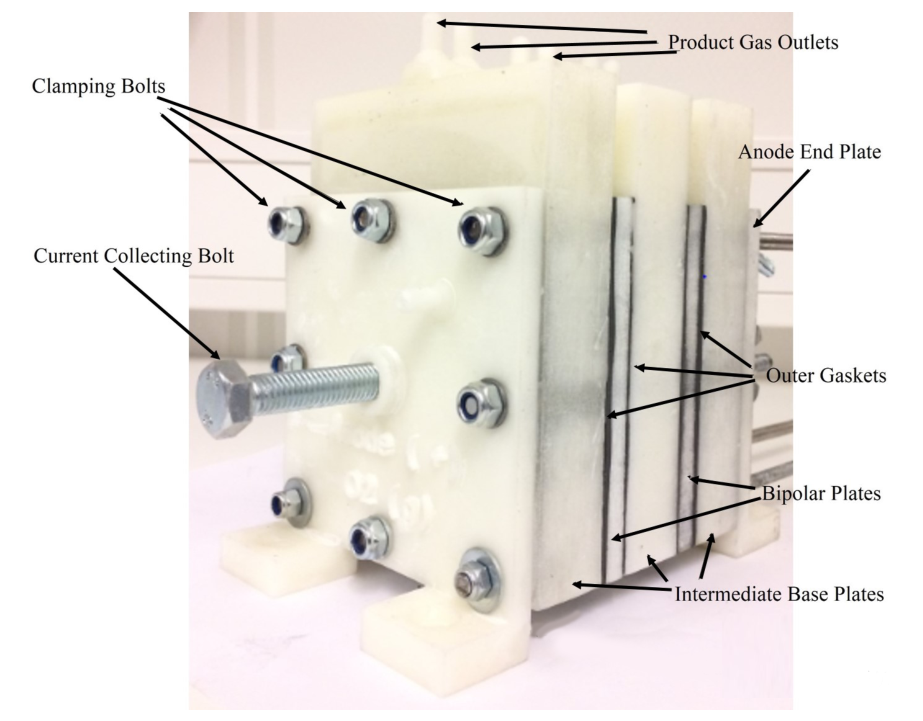
The finished product of monopolar alkaline electrolytic stack is shown in the figure below
2.1 Solidwork design of bipolar alkaline electrolytic reactor
The design and manufacturing process of bipolar alkaline electrolytic reactor is more complex than that of monopolar electrolytic reactor. In order to improve the electrolysis efficiency and the product gas flow rate, a small amount of improvement has been made to the current collector plate, which is composed of three monopole units.
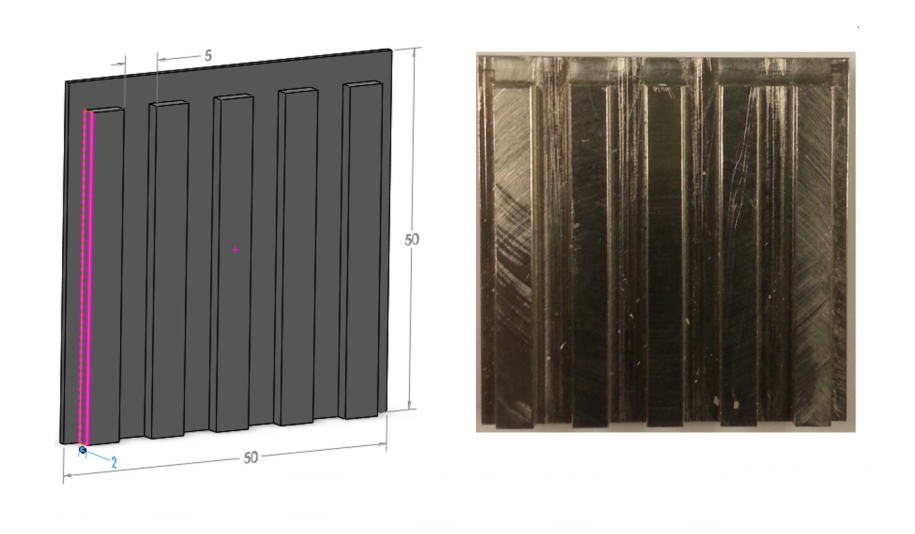
In a bipolar structure, the electrolyte should be kept between the anode and cathode. Therefore, the bipolar plate area must not be affected by the electrolyte. 1mm thick rubber gasket is used inside the stack to prevent leakage inside the stack. In addition, only one side of the anode and cathode should be in contact with the electrolyte. As discussed in monopolar stacking, stainless steel is used as the collector plate to avoid the corrosion of the collector plate in KOH solution. The maximum thickness of the collecting plate is 7mm, and the processing process of the collecting plate is industrial milling machine. In order to increase the contact area and the volume of intermediate electrolyte, a longitudinal groove with a width of 5mm and a depth of 2mm is designed on the collecting plate (other types of flow channels can be made), as shown in Figure 8 below.
Name: Bekah Zeng
Mobile:+86 18122128824
Whatsapp:+86 18122128824
Email:Bekah@thinkru.com
Name: Stella Luo
Mobile:+86-15013165672
Whatsapp:+86-15013165672
Email:stella@thinkru.com
Name: Sally He
Mobile:+86-15626431755
Whatsapp:+86-15626431755
Email:sally.he@thinkru.com
Add:2203, Building 12, No.400 Xincheng Avenue, Zengcheng District, Guangzhou City, China