Knowledge Of Electrochemical Hydrolysis
 e Of Electrochemical Hydrolysis
e Of Electrochemical Hydrolysis
The low-temperature water electrolyzer can be divided into alkaline system and acidic system. They are further divided into limited-gap and zero-gap electrolytic cells (as shown in the figure below). The schematic diagram shows the principle of a single WE electrolytic cell, while the actual system consists of the assembly of many electrolytic cells, called electrolytic stacks. The anode and cathode in WE are separated by separator (diaphragm) to avoid mixing of H2 and O2 gas.
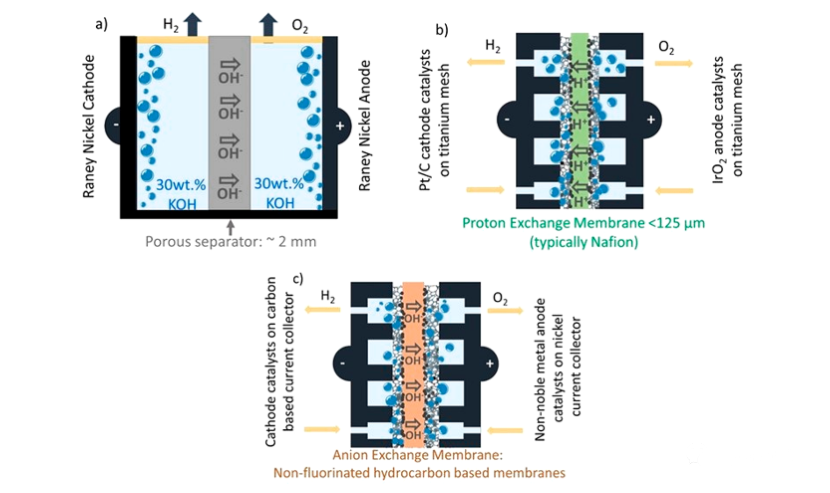
Schematic diagram of three WEs:
(a) Conventional alkaline limited gap WE (AWE).
(b) Zero gap PEMWE using H+conductive film operating under acidic conditions.
(c) Zero gap AEMWE using OH − conductive film. Its goal is to use a noble metal-free catalyst as the cathode and anode of AEMWE.
The technical terms "limited gap" and "zero gap" are related to the separation distance between anode and cathode where O2 precipitation reaction (OER) and H2 precipitation reaction (HER) occur. The limited gap alkaline WE uses porous baffles and aqueous solutions, for example, wt is 30% KOH is a conductive solution, (Figure a). This is a proven technology that has been deployed on a MW scale since the late 1950s. A well-known advantage under alkaline conditions (especially pH>13) is that, unlike acidic media requiring platinum group metal catalysts, non-platinum group metal (non-pGM) based catalysts have good stability for OER and HER. Generally, Renee nickel electrode with high surface area is used for infinite gap alkaline electrolytic cell. The use of porous diaphragms such as Zircon and Perl UTP 500 requires a large distance (>2mm) between the anode and cathode to reduce the crossing of H2 and O2 gases. However, since the ionic resistance is directly dependent on the electrolyte thickness, the large distance is accompanied by high ohmic resistance. It also limits the maximum current density (jmax) that can be reached.
Generally, the current density value of finite gap alkaline WE is 0.25 A/cm2, which is too low for the coupling integration with renewable energy (such as wind energy). Renewable energy needs ES technology that can accept the current density in the range of several A/cm2 and fast dynamic response. A new WE design is being developed, which includes an electrode with a minimum or even zero gap between the electrode and the separator (diaphragm). An example of exploration is the alkali doped ion solvation membrane combined with, for example, wt24% KOH electrolyte. The single cell test using KOH doped ionic solvation film and Raney nickel electrode produced a low battery voltage of 1.8V at a current density of 1.7A/cm2.
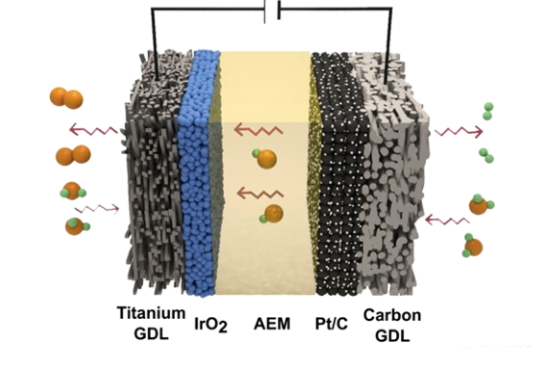
The zero-gap WE design reduces the internal resistance due to the use of low H2 and O2 crossing polymer film. Proton exchange membranes (PEMs, also known as cation exchange membranes) and anion exchange membranes (AEMs) are used for acidic (above b) and alkaline (above c) zero-gap WEs, respectively. Therefore, the zero gap WE will obtain higher current density value than the finite gap electrolytic cell.
For commercial PEMWE, use thin to 50 − 200 μ At PEM of m, the current density value is 1~3 A/cm2, and the service life can reach 15000 − 20000 hours. PEMWE is much more mature than AEMWE. This is related to the fact that the stability of PEM (usually composed of perfluorosulfonic acid under the trademark of Nafion and Aquivion) is significantly higher than that of anion exchange membrane (AEM), although the stability of Nafion is limited to 80 ° C operation. In fact, PEMWE using Nafion diaphragm (electrolyte) usually operates at 60 ° C. Only in recent years, the AEMWE single battery has operated in the electric density range of several A/cm2. Many achievements have been made in improving the stability of AEM, but the durability and performance still need to be further proved.
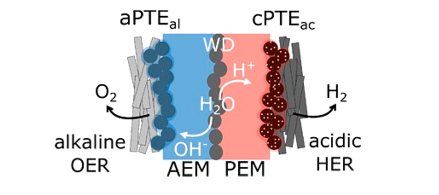
Recent developments in the field of bipolar membranes (BPMs) have opened up new opportunities. The main purpose of BPM is to combine the advantages of PEM and AEM systems. The latter uses low-cost cathode materials (alkaline medium) and active cathode catalysts (acidic medium). In the BPM system, the cation exchange membrane (CEM) and AEM directly contact to form a bipolar interface (as shown in the figure below). The hydrolytic dissociation or water composite catalyst is added between the two membranes to improve the performance. The activity of this double-layer catalyst has been shown to be close to that of the basic HER catalyst.
Schematic diagram of three WEs:
(a) Conventional alkaline limited gap WE (AWE).
(b) Zero gap PEMWE using H+conductive film operating under acidic conditions.
(c) Zero gap AEMWE using OH − conductive film. Its goal is to use a noble metal-free catalyst as the cathode and anode of AEMWE.
The technical terms "limited gap" and "zero gap" are related to the separation distance between anode and cathode where O2 precipitation reaction (OER) and H2 precipitation reaction (HER) occur. The limited gap alkaline WE uses porous baffles and aqueous solutions, for example, wt is 30% KOH is a conductive solution, (Figure a). This is a proven technology that has been deployed on a MW scale since the late 1950s. A well-known advantage under alkaline conditions (especially pH>13) is that, unlike acidic media requiring platinum group metal catalysts, non-platinum group metal (non-pGM) based catalysts have good stability for OER and HER. Generally, Renee nickel electrode with high surface area is used for infinite gap alkaline electrolytic cell. The use of porous diaphragms such as Zircon and Perl UTP 500 requires a large distance (>2mm) between the anode and cathode to reduce the crossing of H2 and O2 gases. However, since the ionic resistance is directly dependent on the electrolyte thickness, the large distance is accompanied by high ohmic resistance. It also limits the maximum current density (jmax) that can be reached.
Generally, the current density value of finite gap alkaline WE is 0.25 A/cm2, which is too low for the coupling integration with renewable energy (such as wind energy). Renewable energy needs ES technology that can accept the current density in the range of several A/cm2 and fast dynamic response. A new WE design is being developed, which includes an electrode with a minimum or even zero gap between the electrode and the separator (diaphragm). An example of exploration is the alkali doped ion solvation membrane combined with, for example, wt24% KOH electrolyte. The single cell test using KOH doped ionic solvation film and Raney nickel electrode produced a low battery voltage of 1.8V at a current density of 1.7A/cm2.
The zero-gap WE design reduces the internal resistance due to the use of low H2 and O2 crossing polymer film. Proton exchange membranes (PEMs, also known as cation exchange membranes) and anion exchange membranes (AEMs) are used for acidic (above b) and alkaline (above c) zero-gap WEs, respectively. Therefore, the zero gap WE will obtain higher current density value than the finite gap electrolytic cell.
For commercial PEMWE, use thin to 50 − 200 μ At PEM of m, the current density value is 1~3 A/cm2, and the service life can reach 15000 − 20000 hours. PEMWE is much more mature than AEMWE. This is related to the fact that the stability of PEM (usually composed of perfluorosulfonic acid under the trademark of Nafion and Aquivion) is significantly higher than that of anion exchange membrane (AEM), although the stability of Nafion is limited to 80 ° C operation. In fact, PEMWE using Nafion diaphragm (electrolyte) usually operates at 60 ° C. Only in recent years, the AEMWE single battery has operated in the electric density range of several A/cm2. Many achievements have been made in improving the stability of AEM, but the durability and performance still need to be further proved.
Recent developments in the field of bipolar membranes (BPMs) have opened up new opportunities. The main purpose of BPM is to combine the advantages of PEM and AEM systems. The latter uses low-cost cathode materials (alkaline medium) and active cathode catalysts (acidic medium). In the BPM system, the cation exchange membrane (CEM) and AEM directly contact to form a bipolar interface (as shown in the figure below). The hydrolytic dissociation or water composite catalyst is added between the two membranes to improve the performance. The activity of this double-layer catalyst has been shown to be close to that of the basic HER catalyst.
 e Of Electrochemical Hydrolysis
e Of Electrochemical Hydrolysis