Membrane Electrode Assembly, The Core Of Hydrogen Fuel Cells
1. What is a Membrane electrode assembly?
Membrane electrode assembly is the core component of proton exchange membrane fuel cell, which provides microchannels for multiphase material transfer and electrochemical reaction sites for PEMFC.
In order to achieve the commercialization goal of fuel cell, MEA with high power density, low Pt load and good durability needs to be prepared. Besides catalysts, the structure of functional layers and the interface between layers have important effects on the performance of MEA.
2.Principle
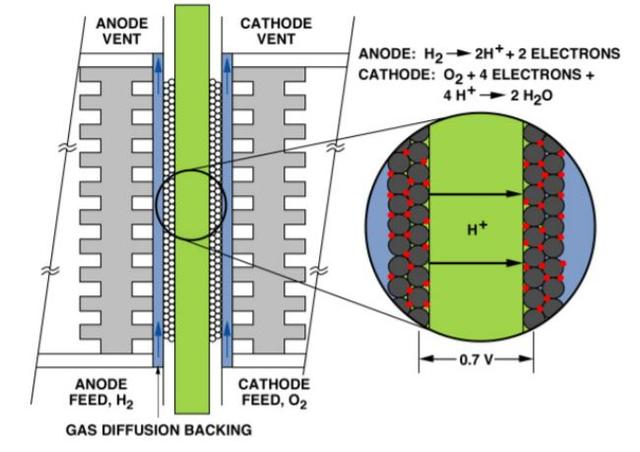
Hydrogen reaches the anode through the gas flow field on the anode plate, reaches the anode catalytic layer through the diffusion layer on the anode, and is adsorbed on the anode catalytic layer. Hydrogen is decomposed into two hydrogen ions, namely proton H+, under the catalytic action of the catalyst platinum, and two electrons are released. This process is called the anodic oxidation of hydrogen, and the reactions occurring on the anode are H2=2H++2e-
At the other end of the battery, oxygen or air passes through the gas flow field on the cathode plate to reach the cathode, through the diffusion layer on the cathode to reach the cathode catalytic layer, and adsorbs to the cathode catalytic layer. At the same time, hydrogen ions pass through the electrolyte to reach the cathode, and electrons pass through the external circuit to reach the cathode. Under the action of cathode catalysts, oxygen reacts with hydrogen ions and electrons to produce water. This process is called cathode reduction of oxygen. The reaction on the cathode is: ½ O2+2H++ 2e-= H2O
The total chemical reaction formula is: H2+ ½ O2=H2O
At the same time, the electrons form a current through the connection of an external circuit, through which power can be output to the load, and the resulting water is discharged with the reaction tail gas through the electrodes.
3.Characteristics of Membrane electrode assembly
High performance membrane electrodes have the following characteristics:
- Can minimize the gas transmission resistance, so that the reaction gas smoothly from the diffusion layer to the catalytic layer for electrochemical reaction.
- Form a good ion channel to reduce the resistance of ion transmission.
- Form good electronic channels.
- Gas diffusion electrode shall ensure good mechanical strength and thermal conductivity.
- The membrane has high proton conductivity.
4.Structure of Membrane electrode assembly
Membrane electrode (MEA) is mainly composed of proton exchange membrane, catalytic layer and gas diffusion layer.
1. Proton exchange membrane
The main function of proton exchange membrane in fuel cells is to realize the rapid proton conduction, and also block the penetration of hydrogen, oxygen and nitrogen between the anode and cathode. The performance of proton exchange membrane directly determines the performance and service life of fuel cells. The ideal proton exchange membrane should have the characteristics of high proton conductivity, low electron conductivity, low gas permeability, good chemical, electrochemical and thermal stability.
Proton exchange membranes are classified according to the fluorine content, including perfluorinated polymer proton exchange membranes, partially fluorinated polymer proton exchange membranes and non-fluorinated polymer proton exchange membranes.
Due to the polytetrafluoroethylene structure of perfluorosulfonic acid polymer, its carbon-fluorine bond has high bond energy, good mechanical properties, chemical stability and thermal stability, and its service life is better than that of other membrane materials. At the same time, due to the presence of hydrophilic sulfonic acid groups on the molecular branch chain, which has excellent ion conduction characteristics, perfluorosulfonic acid membrane has become the mainstream proton exchange membrane scheme.


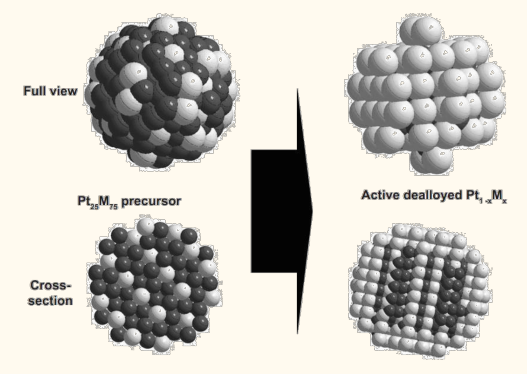
2.Catalyst
The catalytic layer is an important part of the membrane electrode. The anode uses the catalyst to promote the oxidation reaction of hydrogen gas, involving oxidation reaction, gas diffusion, electron movement, proton movement, water migration and other processes. The cathode uses catalyst to promote oxygen reduction reaction, involving oxygen reduction, oxygen diffusion, electron movement, proton movement, reaction generated water discharge, etc.
A good catalyst should have good catalytic activity, high proton conductivity, high electron conductivity and good water management ability, gas diffusion ability.

3. Gas diffusion layer
The two porous gas diffusion layers sandwiched the membrane electrode assembly in the middle, and the main functions included supporting the catalytic layer, collecting the current, conducting the gas and discharging the reaction product water.
The ideal gas diffusion layer is characterized by high conductivity, porosity, proper hydrophilic/hydrophobic balance, high chemical stability, high thermal stability and low cost.
The gas diffusion layer is composed of a support layer and a microporous layer. The support layer materials are mainly porous carbon fiber paper, carbon fiber woven fabric, carbon fiber non-woven fabric and carbon black paper. The microporous layer is usually composed of conductive carbon black and hydrophobic agent.


4.MEA preparation technology
Membrane electrode is composed of proton exchange membrane, catalyst layer, frame and diffusion layer, so how it changed from raw material to finished product, the two main processes are film preparation and catalyst coating.
At present, CCM method (direct coating technology of Catalyst Coated Membrane) is the most widely used MEA preparation method. The catalyst is coated on both sides of the proton exchange membrane, and then the gas diffusion layer and the proton exchange membrane attached with the catalytic layer are combined by hot pressing method.
The preparation process of CCM increased the contact area between the catalyst and the proton exchange membrane, reduced the impedance between the proton exchange membrane and the catalyst, and improved the performance of the membrane electrode.










